In the previous article, we explored the fundamental definition, origin, and core concept of current efficiency. As the second installment of this series, this piece will delve deeper into an explanation of the factors influencing electrode current efficiency.
The full text is 1,243 characters long, with an estimated reading time of 4 minutes.
1.What is Current Efficiency?
1.1 Faraday's Law
1.2 Electrochemical Equivalent
1.3 Current Efficiency
2.Factors Affecting Electrode Current Efficiency
2.1 Anodic Current Efficiency of Chlor-Alkali Electrolyzers
2.2 Anodic Current Efficiency of Sodium Hypochlorite Production by Electrolysis
2.3 Cathodic Current Efficiency of Metal Electrodeposition from Aqueous Solutions
3.Factors Affecting Current Efficiency of Electrochemical Reactors
3.1 Counter Electrode Side Reactions and Electrolyte Side Reactions
3.2 Design Factors of Electrochemical Reactors
The electrode is a critical factor affecting the current efficiency for target product yield. A direct cause of reduced electrode current efficiency lies in the electrochemical side reactions occurring on the electrode that are irrelevant to the formation of the target product—here specifically referring to the side reactions potentially accompanying the target electrochemical reactions on the electrode surface. Below, we illustrate the factors influencing electrode current efficiency through three typical cases: chlor-alkali electrolysis, electrolytic production of sodium hypochlorite, and aqueous metal electrodeposition.
In the chlor-alkali industry, ruthenium-titanium mixed metal oxide coated titanium electrodes are adopted as anodes. The desired primary reaction at the anode is chlorine evolution, and the anodic chlorine evolution efficiency typically maintains above 90% over the long term. However, a certain proportion of oxygen evolution reactions are inevitable, resulting in higher power consumption for chlorine production than the theoretical value. Therefore, in coating design, it is usually necessary to suppress the oxygen evolution reaction of the electrode as much as possible by regulating the relative changes between the oxygen evolution overpotential and the chlorine evolution overpotential. Common measures include controlling the ruthenium-titanium ratio and introducing other doping elements.
Figure 1 illustrates the effect of the ruthenium-titanium ratio on the oxygen content in the gas and the anodic oxygen evolution overpotential. It indicates that a higher proportion of ruthenium oxide corresponds to a lower oxygen evolution overpotential, which in turn leads to a higher oxygen content in the electrolytic cell.
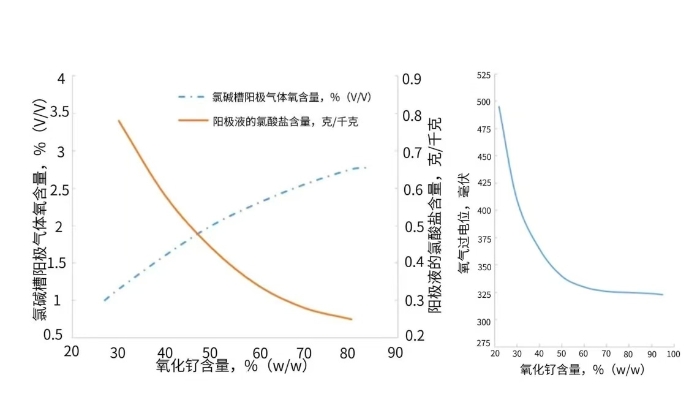
Figure 1. Effect of Ruthenium Oxide Content on Gas Oxygen Content (Left) and Oxygen Evolution Overpotential (Right)
Although oxygen evolution side reactions mainly occur on the anode surface, the design of the anode coating itself is not the sole critical factor. Operating current density, temperature, electrolyte composition, and the stable supply of sufficient chloride ions on the electrode surface are all key factors affecting chlorine evolution efficiency.
For instance, reducing the current density, increasing the electrolyte temperature, and decreasing the sodium chloride concentration in the electrolyte will all lead to an increase in the oxygen content in the chlorine gas.
Therefore, in coating development, it is necessary to consider increasing the electrode's oxygen evolution potential to inhibit oxygen evolution reactions. Meanwhile, in electrolyzer design, full account must be taken of operating conditions and mass transfer to ensure an adequate supply of reactive ions on the electrode surface and the timely detachment of products from the electrode surface, thereby avoiding the formation of solution dead zones.
In the design of chlor-alkali electrolyzers, it has been found that under the conditions of the same surface area and other parameters, expanded mesh metal anodes generate more oxygen than flat-plate or narrow-blade structured anodes (as shown in Figure 2). This phenomenon may be related to the coating smoothness of different structural forms, as coating smoothness has been proven to affect the kinetics of oxygen evolution reactions.
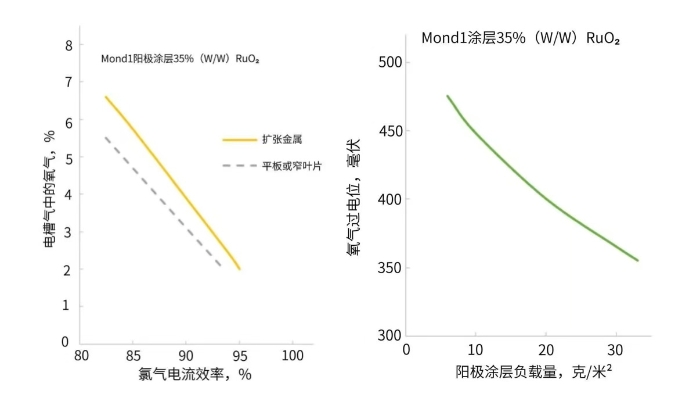
Effects of Anode Shape on Chlorine Current Efficiency and Oxygen Percentage in Chlor-Alkali Diaphragm Cells (Left) and Relationship Between Anode Coating Loading and Anodic Oxygen Evolution Overpotential (Right)
The electrolytic production of sodium hypochlorite generally adopts the diaphragm - free electrolysis process. Its operating parameters such as temperature, current density, and brine concentration are all significantly lower than those of chlor - alkali electrolyzers. The anode coating used is similar to that in chlor - alkali electrolysis. Under normal operating conditions, the anodic chlorine evolution current efficiency can always reach over 90%. However, due to the substantial differences in electrolyzer design and application scenarios between this process and chlor - alkali electrolysis, in addition to the common influencing factors such as brine concentration and current density, there exists a unique influencing factor on anode current efficiency that is different from chlor - alkali electrolysis, namely temperature.
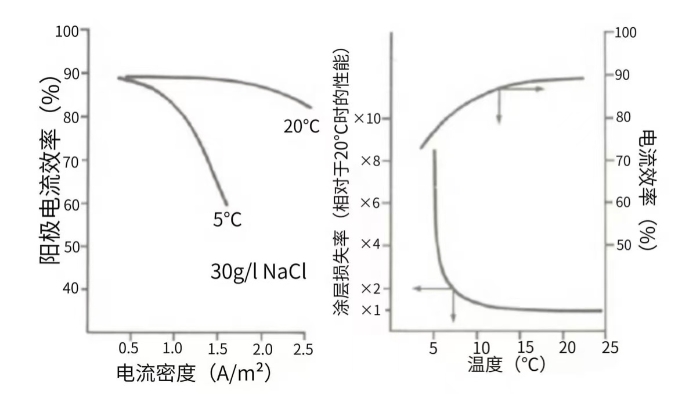
Figure 3 Chlorine Evolution Efficiency of Conventional Electrode Coatings for Electrolytic Sodium Hypochlorite Production at Different Temperatures (Left); Variations of Coating Consumption Rate and Chlorine Evolution Efficiency with Temperature under Conditions of 1000 A/m² Current Density and 30 g/L NaCl Concentration (Right)
As shown in the left panel of Figure 3, when the temperature drops to 5°C, the chlorine evolution efficiency of the electrode decreases sharply with the increase of current density. Two potential causes for this phenomenon have been identified so far. Firstly, the observed electrode potentials for chlorine and oxygen evolution exhibit significantly different temperature sensitivities. Specifically, the chlorine evolution potential rises much faster than the oxygen evolution potential at low temperatures, resulting in the oxygen evolution potential being lower than the chlorine evolution potential under low-temperature conditions and a consequent increase in oxygen evolution current efficiency. In addition, at temperatures below 10°C, the formation of hydrates on the anode surface may create a diffusion barrier, which further leads to local chlorine depletion and a subsequent reduction in chlorine evolution efficiency. A direct consequence of this phenomenon is the rapid increase in coating consumption rate. As illustrated in the right panel of Figure 3, the consumption rate of conventional coatings at 7.5°C is twice that at 20 - 25°C. A further decrease in temperature will trigger a sharp rise in coating consumption rate. Therefore, customers are advised to opt for customized low-temperature chlorine evolution coatings when encountering such application conditions.
Aqueous electroplating and electrowinning primarily occur at the cathode. The main factor influencing their current efficiency is the proportion of the hydrogen evolution reaction that competes with the metal electrodeposition reaction on the cathode surface. Which of these two competing reactions—hydrogen evolution and metal ion deposition—predominates depends, on the one hand, on the equilibrium potentials of their respective electrochemical reactions. On the other hand, it is also determined by the electrolyte composition (such as pH value and metal ion concentration) and operating conditions (current density). Additionally, the preferential deposition of impurity ions can also impair the cathodic current efficiency.
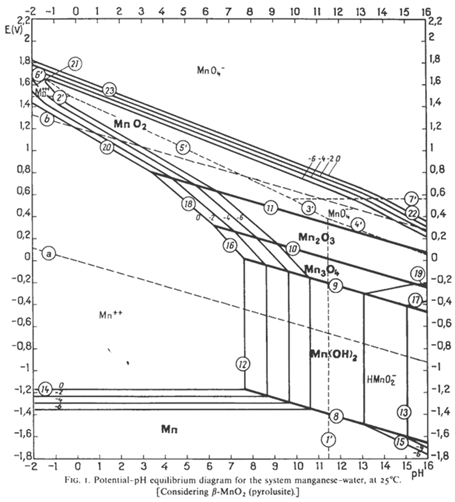
Figure 4 Pourbaix Diagram of the Mn-H₂O System
Taking manganese electrowinning as an example, Figure 4 presents the Pourbaix diagram of the Mn-H₂O system. According to the data in Figure 4, the reduction potential of divalent manganese ions is thermodynamically lower than the equilibrium potential of hydrogen evolution. However, the reaction for metallic manganese deposition is diffusion-controlled, while the hydrogen evolution reaction is electrochemically controlled. In industrial manganese electrolysis processes, two key measures are typically adopted: on one hand, increasing the manganese ion concentration in the cathodic region to enhance the ion concentration on the cathode surface and reduce the overpotential for manganese deposition; on the other hand, adding aqueous ammonia to the cathodic region to adjust its pH to the range of 7 - 8. This pH adjustment not only increases the hydrogen evolution overpotential to inhibit the hydrogen evolution side reaction but also prevents the formation of hydroxides by limiting the pH to no more than 8.
The addition of ammonia thermodynamically elevates the cathodic manganese deposition potential and kinetically increases the solution pH, which further raises the hydrogen evolution overpotential. The combined effects of these thermodynamic and kinetic modifications significantly contribute to the improvement of current efficiency. Literature studies have demonstrated that, under otherwise identical conditions, adjusting the pH of the cathodic solution from 6.969 to 7.9225 by adding aqueous ammonia can increase the current efficiency of manganese electrowinning from 61.6% to 79.1%.
In the field of zinc electrowinning, the impact of impurity ions on current efficiency can be extremely severe under certain circumstances. Typically, the zinc sulfate solution obtained after purification still contains impurities that adversely affect current efficiency. The primary hazards of these impurities lie in promoting hydrogen evolution at the cathode and causing a positive shift in the cathode potential. Due to the relatively high deposition overpotential of zinc, this phenomenon accelerates both the anodic dissolution of cathodic zinc and hydrogen evolution, thereby reducing current efficiency. Impurities affecting the current efficiency of zinc electrowinning are classified into three groups based on their hazard levels (from least to most severe): Group 1 - Pb, Cd, Fe, Ag; Group 2 - Ni, Co, Cu; and Group 3 - Ge, Te, Se, Sb, As.
Under normal industrial concentration levels, impurities such as Pb, Cd, Fe, and Ag have negligible effects on current efficiency. When the concentrations of Ni, Co, Cu, etc., are kept below 5 - 10 mg/L, they do not significantly reduce current efficiency. In contrast, Ge, Te, Se, Sb, and As exert the most drastic negative impact on current efficiency. The coexistence of multiple impurities can alter the individual effects of each impurity, intensifying their overall hazard and leading to a substantial drop in current efficiency. Therefore, intensive purification of the electrolyte is essential for zinc electrowinning to minimize the concentration of impurity ions as much as possible.
Typically, an increase in current density results in a decrease in ion concentration near the cathode, which favors hydrogen evolution and hinders metal deposition, consequently reducing the current efficiency for metal deposition. Nevertheless, there are special cases where higher current density can improve current efficiency. For instance, in hexavalent chromium electroplating, elevated current density disrupts the local colloidal film on the cathode surface, thereby accelerating chromium deposition and inhibiting the hydrogen evolution side reaction.
As an additional note, in accordance with Faraday's laws of electrolysis, the actual current efficiency is generally less than 100%. However, the apparent current efficiency may exceed 100% under specific conditions. This phenomenon is typically attributed to the increased production of target products caused by supplementary chemical reactions, and it does not imply the invalidation of Faraday's efficiency principle.
In electrochemical reactors, there are more factors influencing current efficiency. The primary ones include side reactions occurring on the electrodes, side reactions in the electrolyte, and the design of the electrochemical reactor itself. The middle section has illustrated the factors affecting electrode current efficiency by taking chlor-alkali electrolysis, electrolytic sodium hypochlorite production, and aqueous metal electrodeposition as examples. In the following section, we will analyze the factors influencing current efficiency in electrochemical reactors through specific cases. Stay tuned for more insights!