The use of oxidative chlorine for chlorination disinfection of tap water began in the United States in 1908. Prior to this, due to the lack of effective sterilization methods, there were multiple major epidemics
caused by the spread of pathogens through drinking water. After the introduction of chlorination disinfection, a notable phenomenon in the United States was the sharp decline in the annual mortality rate caused by typhoid fever: the number of deaths per million people dropped rapidly from 300 to almost zero by 1950, demonstrating the significant impact of chlorination disinfection on human survival and health. To this day, chlorination disinfection remains the most common disinfection method for tap water.
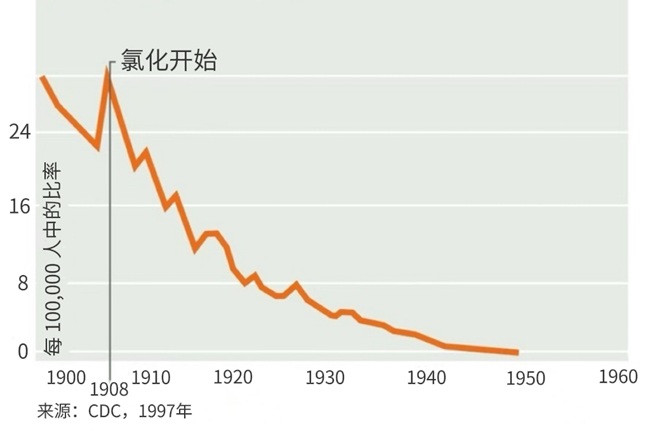
Figure 1. Historical Mortality Rate Due to Typhoid Fever in the United States
From a microbiological perspective, drinking water that has undergone chlorination disinfection is safe because pathogens are either killed or rendered incapable of reproduction, thus being unable to infect human hosts. But how does chlorine exert its disinfection effect to make water safe for drinking? When chlorine is added to water, two chemical substances are formed, collectively known as free chlorine. These substances—hypochlorous acid (HOCl, electrically neutral) and hypochlorite ion (OCl⁻, electronegative)—behave quite differently. Hypochlorous acid is not only more reactive than the hypochlorite ion but also a stronger disinfectant and oxidizing agent. Although the hypochlorite ion is less reactive, sufficient bactericidal activity and disinfection effects can be achieved with a longer contact time.
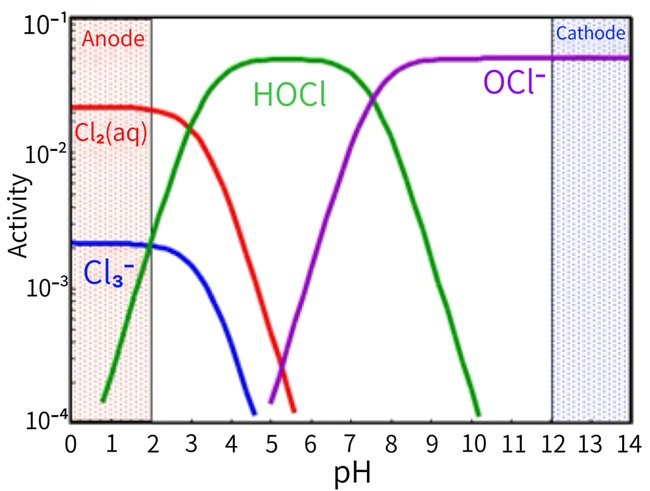
Figure 2. Predominant Forms of Available Chlorine at Different pH Levels
The concentration ratio of hypochlorous acid molecules to hypochlorite ions in water is determined by pH value. When the pH is low (below 7.5), hypochlorous acid molecules predominate, while at high pH levels (slightly above neutral), hypochlorite ions become the dominant species. Consequently, the rate and efficacy of chlorination disinfection are affected by the pH of the water being treated. Fortunately, bacteria and viruses remain relatively susceptible to chlorination across most pH ranges.
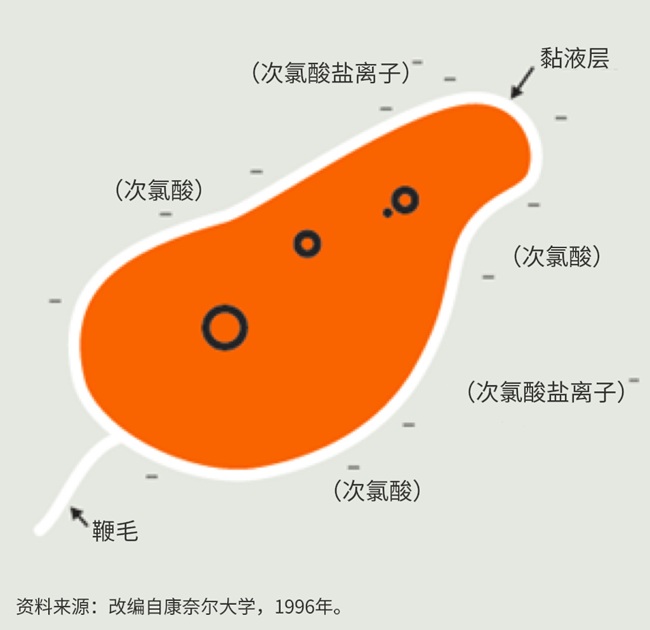
Figure 3. Schematic Diagram of the Bactericidal Efficacy of Available Chlorine in Different Forms
An additional reason for hypochlorous acid's superior bactericidal effect compared to hypochlorite ions is that bacterial pathogens typically carry a natural negative charge on their surfaces. Consequently, the electrically neutral hypochlorous acid molecules can penetrate these surfaces more readily than the negatively charged hypochlorite ions, resulting in stronger disinfecting capability of hypochlorous acid molecules.
Since tap water has a neutral pH value, the more stable hypochlorite ions are the species responsible for exerting the disinfection effect in chlorination. However, hypochlorite ions may decompose in water through the following reactions:
ClO⁻+H₂O→OH⁻+HClO
(1.1)
ClO⁻+2HClO→ClO₃⁻+2HCl
(1.2)
3ClO⁻→ClO₃⁻+2Cl⁻
(1.3)
Reactions 1.1, 1.2, and 1.3 collectively represent the potential chemical decomposition of sodium hypochlorite, and the primary oxygen-containing chlorine salt among its decomposition products is sodium chlorate. The extent to which the above decomposition reactions proceed mainly depends on factors such as sodium hypochlorite concentration, temperature, and time. A higher concentration accelerates the reaction; a higher temperature is more favorable for the reaction; and a longer duration allows the reaction to proceed more fully. Among these, concentration and temperature are the two most critical influencing factors. The aforementioned reactions proceed vigorously at temperatures above 40°C and lead to a significant decrease in pH. To inhibit these side reactions, the pH of sodium hypochlorite solutions requiring long-term storage must be maintained above 11. Among them, the disproportionation equilibrium constant of hypochlorite ions in Reaction 1.3 is extremely large, and the reaction efficiency depends on temperature and concentration.
ClO⁻+O₂→ClO₃⁻
(1.4)
Reaction 1.4 represents the potential reaction between hypochlorite ions and oxygen under light conditions, with chlorate ions also being its primary product. For commercial sodium hypochlorite aqueous solutions, studies have indicated that after two years of storage, the concentration of sodium chlorate can reach an astonishing 260 g/L at maximum. According to China's national standard GB 5749-2022 Standards for Drinking Water Quality, the concentration of chlorate in drinking water must not exceed 0.7 mg/L, and it is mandatory to test this value when using sodium hypochlorite for disinfection. Regulations from the American Water Works Association (AWWA)—an authoritative institution in global safe water supply—regarding the application of commercial sodium hypochlorite solutions for drinking water disinfection include the following: a. Sodium hypochlorite solutions should be used as soon as possible after production, and the production date must be clearly marked; b. The concentration of sodium hypochlorite in the solution should be kept as low as possible during use and storage. Diluting the solution by half can reduce the perchlorate concentration during storage to 1/7 of that before dilution; c. The pH value of sodium hypochlorite solutions during storage should be controlled within the range of 11-13. A pH below 11 facilitates the formation of chlorate, while a pH above 13 promotes the formation of perchlorate; d. Impurity metal ions in the solution should be removed as much as possible; e. The storage temperature should be kept below 15℃ as much as possible. For every 5℃ decrease in temperature, the decomposition rate of sodium hypochlorite can be reduced by 50%; f. The production specifications of sodium hypochlorite should be strictly controlled to avoid the formation of chlorate during the production process.
China's national standard GB 5749-2022 Standards for Drinking Water Quality stipulates that when sodium hypochlorite is used for disinfecting standard tap water, the dosage is generally 1-3 mg/L. If the water contains a large amount of algae and has poor water quality, the dosage can be increased to 3-5 mg/L. This concentration is already very low, making decomposition relatively difficult. Therefore, the main decomposition issue lies in the state of sodium hypochlorite before it is dosed.
According to China's National Standard GB 19106-2013 Sodium Hypochlorite, the available chlorine concentration in commercial sodium hypochlorite is classified into three grades, ranging from 5 wt% to 13 wt%. Such high concentrations are highly conducive to the decomposition of sodium hypochlorite. Studies have shown that the initial chlorate content in commercial sodium hypochlorite solutions can reach 5 g/L. To suppress the concentrations of disinfection by-products such as chlorate and chlorite, commercial sodium hypochlorite solutions have an extremely limited shelf life and stringent storage requirements: they need to be diluted before storage (thus occupying more storage space), require precise pH adjustment with a large amount of free alkali, and need to be stored at low temperatures. Therefore, directly producing low-concentration sodium hypochlorite solutions via electrolysis using a sodium hypochlorite generator has inherent advantages over commercial sodium hypochlorite in terms of chlorate by-product control.
In-situ electrolytic production of sodium hypochlorite is divided into the diaphragm-free method and the diaphragm method. For the former, the concentration of sodium chloride in the feed water is usually around 3%, and the available chlorine concentration in the effluent ranges from 0.8% to 1.0%. The latter adopts a structure similar to that of a chlor-alkali electrolyzer to directly generate chlorine gas and sodium hydroxide, which are then mixed outside the electrolyzer to produce sodium hypochlorite. Due to its simple operation, the diaphragm-free method remains the dominant type of electrolytic sodium hypochlorite generator available on the market.
Like commercial sodium hypochlorite, on-site electrolytic production of sodium hypochlorite also has the potential to generate chlorate, but the influencing factors differ significantly. Among these, electrolysis-related factors are the most prominent.
If available chlorine remains in the electrolyte for a relatively long time, hypochlorite ions will have a greater chance of contacting the titanium anode, in which case the following two oxidation reactions may occur to produce chlorate.
ClO− + 2H2O → ClO3− + 4H+ + 4e−
(1.5)
12ClO− + 6H2O → 4ClO3− + 8Cl− + 12 H+ + 3O2 + 12e−
(1.6)
The above two electrolytic reactions are related to electrode potential, and different types of coated titanium anodes have a significant impact on them.
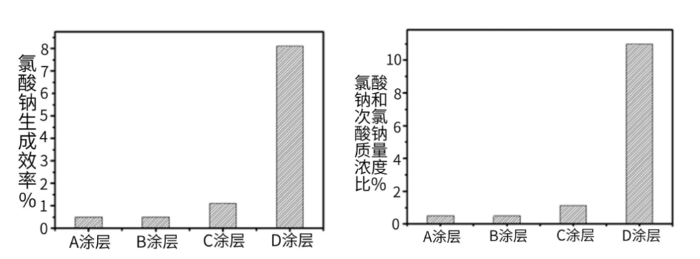
Figure 4. Effects of Different Anode Coatings on the Current Efficiency of Chlorate Formation (Left) and the Mass Ratio of Chlorate to Sodium Hypochlorite Concentrations (Right) (1000 A/m², 28 °C, 3 wt% NaCl)
Based on the data in Figure 4, under the condition that temperature, sodium chloride concentration, anode current density and other parameters remain unchanged, anode coatings have a significant impact on the current efficiency of chlorate by-product formation. The well-performing Coating A results in a chlorate mass concentration of less than 1% of the sodium hypochlorite mass concentration, which represents a substantial advantage over commercial sodium hypochlorite. However, if an inappropriate anode coating (e.g., Coating D) is selected, the formation efficiency of chlorate will be significantly increased, and the mass concentration of chlorate in the effluent can reach more than 10% of that of sodium hypochlorite.
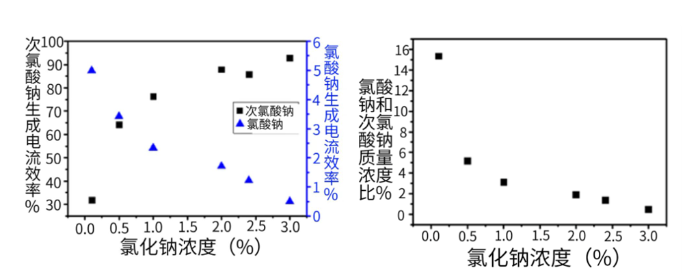
Figure 5. Effects of Different Sodium Chloride Concentrations on the Current Efficiency of Chlorate Formation (MAGNETO Ruthenium-Based Electrode, 1000 A/m², 28 °C)
The concentration of sodium chloride in the feed water also exerts a significant impact on the current efficiency of chlorate formation. When the sodium chloride concentration is low, the electrode voltage becomes relatively high, which on one hand causes a rapid decrease in chlorine evolution efficiency, and on the other hand is more conducive to the oxidation of hypochlorite ions to form chlorate ions. As the sodium chloride concentration decreases from 3.0% to 0.1%, with the anode current density remaining unchanged, the current efficiency of sodium hypochlorite formation drops from 93% to 32%, while the current efficiency of chlorate formation rises from 0.5% to 5%. Eventually, the mass concentration ratio of chlorate to sodium hypochlorite in the effluent increases from nearly 0 to 15.2%. However, sodium hypochlorite generators generally do not reduce the salinity, so the impact of this issue is negligible in practice.
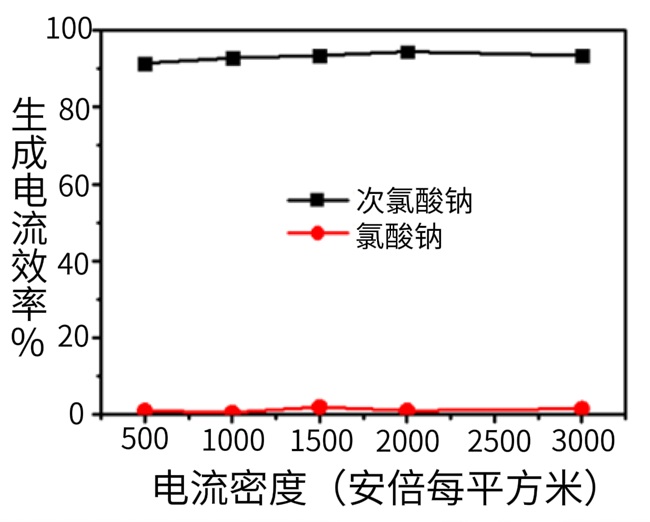
Figure 6. Effects of Current Density on the Current Efficiency of Chlorate Formation (MAGNETO Ruthenium-Based Electrode, 28 °C, 3% NaCl)
As shown in Figure 6, under the normal sodium chloride concentration of 3%, the current density has little effect on the generation of sodium chlorate. The sodium chlorate generation efficiency remains basically unchanged when the current density ranges from 500 A/m² to 3000 A/m².
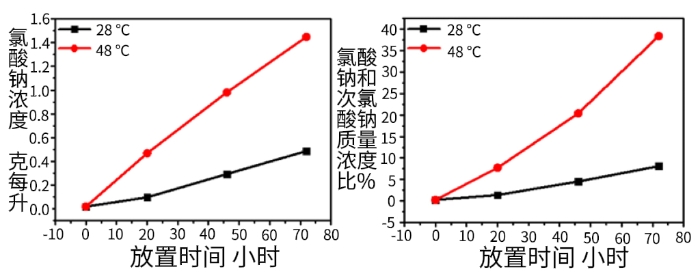
Figure 7 Decomposition of 7.5 g/L Sodium Hypochlorite Solution Stored in the Dark at Different Temperatures
Based on the data in Figure 7, the 7.5 g/L sodium hypochlorite aqueous solution—with a concentration close to that of the sodium hypochlorite aqueous solution produced by on-site diaphragm-free electrolysis—undergoes continuous decomposition within three days when stored in the dark. By titrating to measure changes in sodium chlorate concentration, it was found that sodium hypochlorite is almost entirely converted into sodium chlorate. Therefore, the decline in sodium hypochlorite concentration corresponds to an increase in sodium chlorate concentration and a rapid rise in the sodium chlorate/sodium hypochlorite ratio. After three days at 48°C, the weight ratio of sodium chlorate to sodium hypochlorite reaches as high as 40%.
Compared with commercial sodium hypochlorite, which has a high concentration and requires transportation and long-term storage, on-site electrolytically generated sodium hypochlorite inherently offers advantages in controlling chlorate by-products during tap water disinfection due to its low concentration and on-demand production. However, this method also requires attention to the control of electrolysis conditions, as well as the storage temperature and storage time of the product.
MAGNETO manufactures high-performance electrolytic chlorine-generating anodes, which boast stable chlorine evolution efficiency and long service life, among other advantages. The products have undergone over a decade of customer validation, ensuring reliable and continuous operational performance in industrial applications of electrolytic chlorine generation.


Electrochlorination Brine MMO Plate Anode(Left)Electrochlorination Seawater MMO Mesh Anode(Right)
MAGNETO Electrolytic Chlorine-Generating Electrodes (Selection)