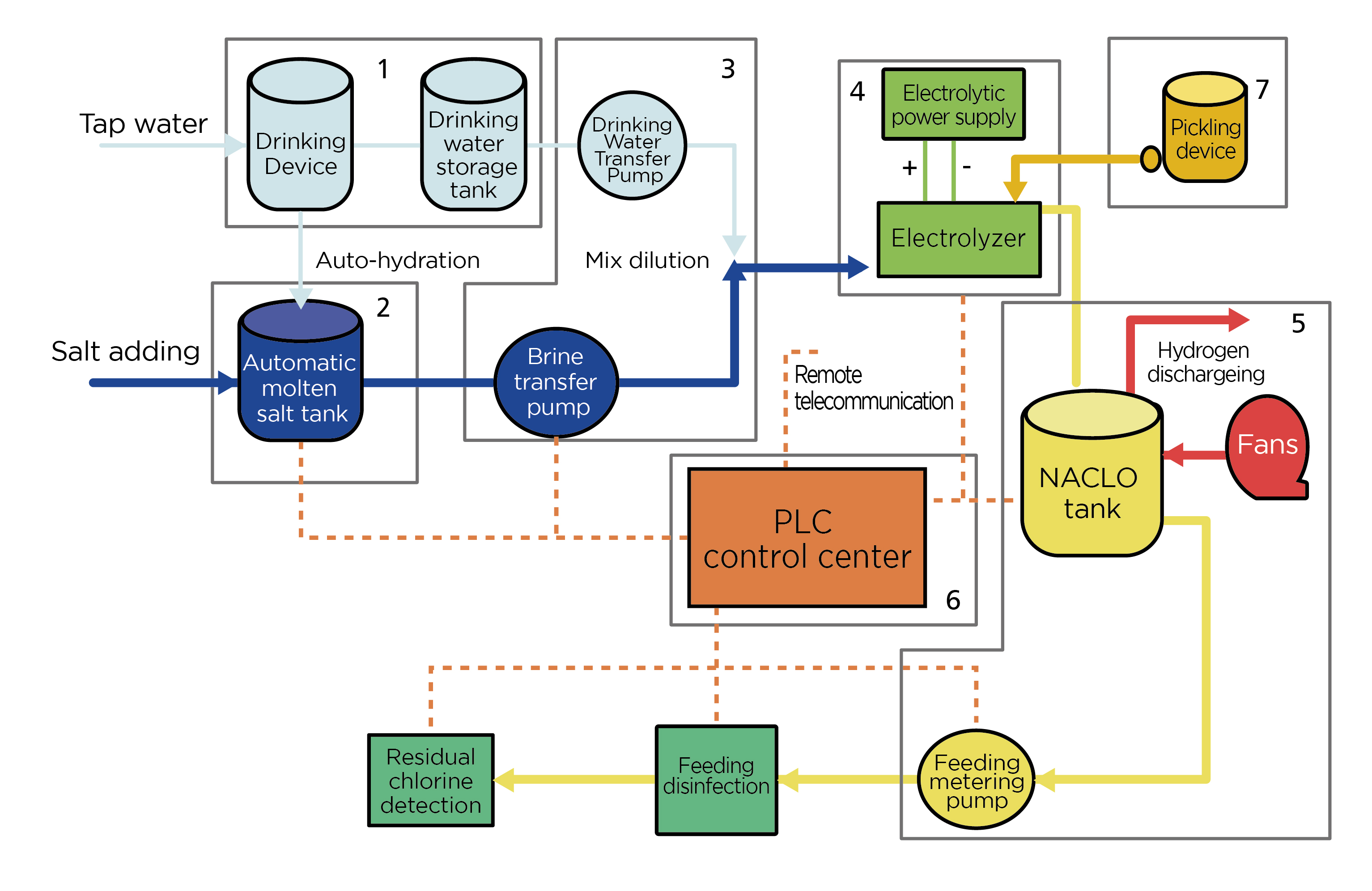
The core part of sodium hypochlorite generator is electrolysis tank, electrode and power supply, supplemented by water softening unit, salt dissolving unit, control unit and other equipment supporting the use. The process has the characteristics of easy to obtain raw materials, stable operation, pure products, etc. It can be widely used in the oxidation of drinking water, industrial circulating water, sewage and other types of water quality, aquariums, farms, public environmental disinfection and other process units.
Sodium Hypochlorite Generator